
2024 European Innovation Score board Released
2024 European Innovation Score board Released Marie LatourSettings The European Commission released the Innovation Scoreboard 2024 on July 8, offering an in-depth comparative analysis of
The funding provided by the EIC Accelerator helps bridge the gap between advanced research and market launch. It provides grant money and equity investment (up to 17.5M€) to support high-risk innovative startups and SMEs to finish their product development and reach the market while supporting the scaling up of their business. Hence, it targets companies that are at a Technology Readiness Level (TRL) 6-8 and aims to help them reach TRL 9, namely ready for commercialization.
The Technology Readiness Level (TRL) scale is valuable a tool for assessing the maturity of technologies that intends to standardize the discussion of technical development across different industries. However, it may not be well suited for the pharmaceutical industry.
Drug development is a highly regulated process that involves expensive and extensive clinical trials before a product can be marketed. Therefore, the TRL scale used for engineering products and technologies, may not reflect the development status of startups in the Life Science industry.
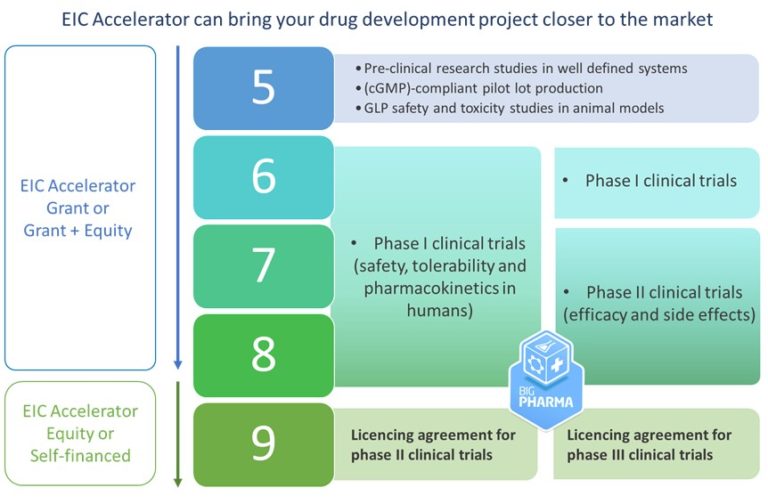
The EIC Accelerator program requirements for pharma startups reflect the unique challenges this industry faces to commercialize a product. Drug development projects must at least have completed the pre-clinical research studies and be in the Clinical Trial Phase I or II in order to be considered TRL 6-8 and eligible for funding. This means they have conducted the initial safety training and are starting to test the efficacy on a larger patient population. The funding provided by the EIC, along with a private pharmaceutical co-investment, can help complete the clinical trials and bring the product closer to a commercial stage or to a licensing agreement.
If you are working on a drug development project and meet the eligibility requirements for the EIC Accelerator programme, this could be the funding opportunity you have been looking for to bring your product to market.
At Euro-Funding we offer support throughout the entire grant identification, processing and justification process. If you have a project and you are looking for grants or subsidies to finance it, contact us and maximize the return of each project.

2024 European Innovation Score board Released Marie LatourSettings The European Commission released the Innovation Scoreboard 2024 on July 8, offering an in-depth comparative analysis of

Strategic partnership with CleanTech Business Club Marie LatourSettings Joins Forces to Propel Sustainable Innovation On the occasion of its 10th anniversary celebrated in Munich on

2024 European Calls for Environmental and Climate Projects Asier PollánSettings WHAT IS THE PURPOSE OF THIS PROGRAMME? The 2024 LIFE calls for proposals opened on
